SELECT trial information
The double-blinded SELECT cardiovascular outcomes trial compared subcutaneous once-weekly semaglutide 2.4 mg with placebo as an adjunct to standard of care for prevention of major adverse cardiovascular (MACE) events over a period of up to 5 years. Read more about its background, methodology, results, and more.
We are still gathering material for this library.
Please check again soon.
We didn't find any resources that match your selection.
Please adjust your filter(s) and try again.

Obesity as a risk factor for ASCVD Video
Obesity as a risk factor for ASCVD Video


SELECT Inclusion Exclusion Criteria and Dosing
SELECT Inclusion Exclusion Criteria and Dosing


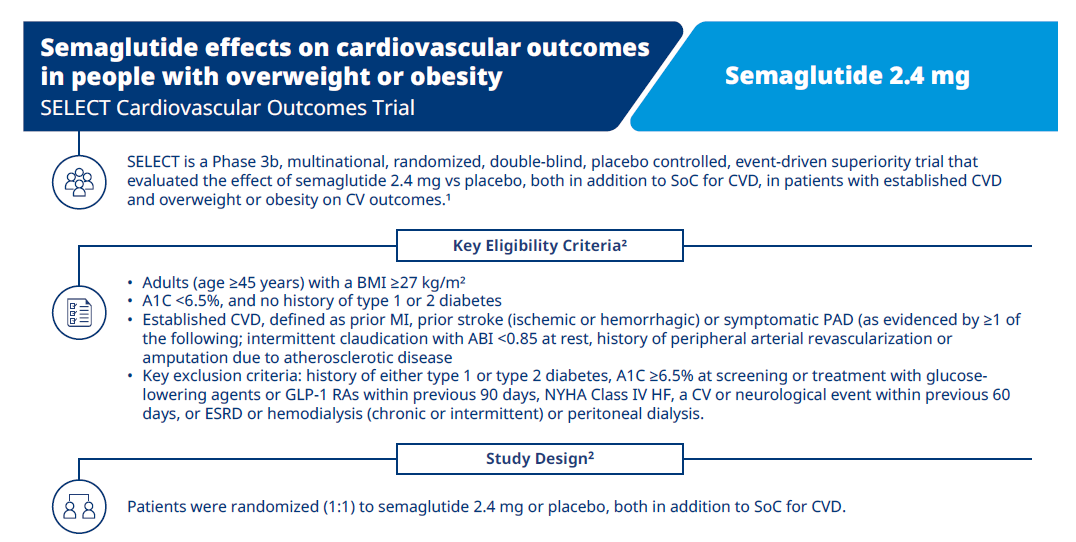
SELECT CVOT overview
Overview of SELECT CVOT trial, design, efficacy and safety results.


Semaglutide s.c. 2.4 mg OW is not indicated for MACE risk reduction. Safety and efficacy are not established for this use under investigation. Semaglutide s.c. 2.4 mg OW is FDA-approved for chronic weight management in adults with obesity (BMI > 30), or overweight (excess weight) (BMI > 27) who also have weight-related medical problems, used with a reduced-calorie meal plan and increased physical activity. Semaglutide s.c. 2.4 mg OW is also indicated for use in patients aged 12 years and older with an initial BMI at or above the 95th percentile for age and sex. Please see the full prescribing information for Semaglutide s.c. 2.4 mg OW here. Novo Nordisk does not recommend or suggest use of its medicines in a manner inconsistent with FDA-approved labeling.
Featured Insights and Resources

Additional Information
Speak to a medical representative
If you are a healthcare provider from the United States, click below to request information directly from the Novo Nordisk Medical Information department. If you are a patient, please visit here to contact us, or call 1-800-727-6500.
Search the Medical Information Database
The Scientific Exchange is a resource for U.S. Healthcare Professionals to learn more about disease states investigated by Novo Nordisk and our related products.
Scientific Exchange is a trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
Non-US Health Care Professionals, please go to www.novonordiskpro.com.
- Terms of Use
- Privacy Statement
- Consumer Health Privacy Notice
- Cookie Notice
- Privacy Request
- Contact Us
- novonordisk-us.com
© 2024 Novo Nordisk All rights reserved.
